HDACs in multiple myeloma
Epigenetic alterations have been shown to offer valuable options for controlling carcinogenesis. Distinct epigenetic events are reported to drive tumor formation, progression, and metastasis. Epigenetic modifications such as DNA methylation, histone acetylation, chromatin remodeling and non-coding RNAs can lead to aberrant gene expression and promote epithelial-mesenchymal transition (EMT), cancer cell stemness and cellular transformation. Histone deacetylases (HDACs) are essential regulators of histone acetylation and some of them are overexpressed in multiple myeloma patients. Among these, HDAC6 is the main player explored in our research. HDAC6 is a cytoplasmic protein able to deacetylate tubulin and regulate microtubule network stability. Moreover, HDAC6 contains a C-terminal ZnF-UBP domain by which it can recruit ubiquitinated proteins and activate the aggresome pathway. Therefore, this domain is thought to be responsible for the acquisition of resistance against proteasome inhibition conventional therapies. Based on this, several chemical and molecular approaches have been designed in our group to study the impact of a selective inhibition of the ZnF-UBP domain in multiple myeloma cells. Moreover, the study of dual proteasome-HDAC inhibitors contributes to assess the potential of a simultaneous inhibition of the proteasome and aggresome pathways in multiple myeloma. The role of other HDACs is also investigated in multiple myeloma cells to describe further interplays and potential compensation mechanisms among isoforms.
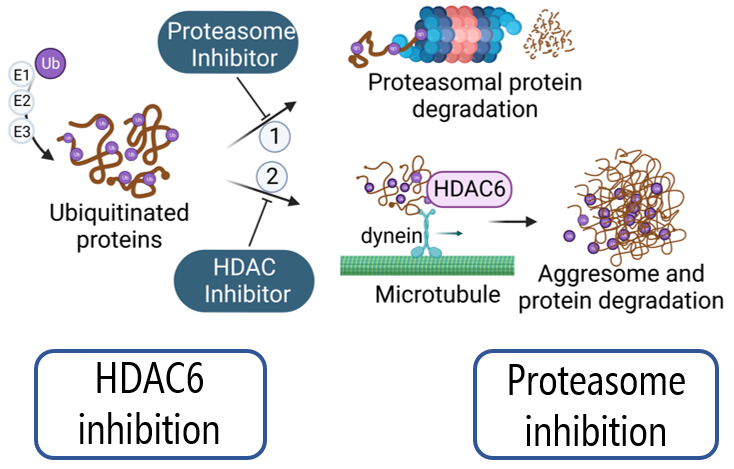
Figure created with BioRender.com